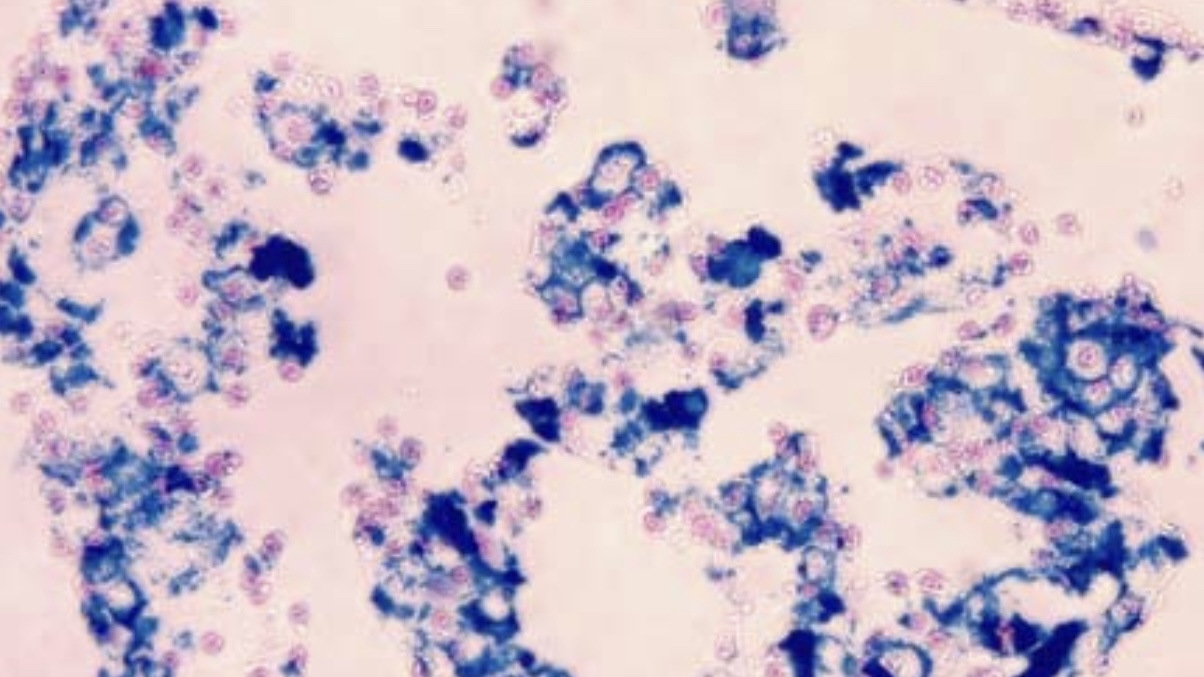
Perl’s Prussian Blue Stain
Perl’s Prussian Blue stain, also known as iron stain, is a widely used histological technique for detecting ferric iron in tissues. It highlights the presence of hemosiderin, a storage form of iron.
Principle
The staining technique is based on the chemical reaction between ferric iron in tissue sections and the acidic ferrocyanide solution used in the stain. This reaction forms an insoluble blue compound, Prussian blue, at the site of the iron. This distinct blue color allows for the visual identification of iron deposits within cells and tissues.
Method of Application
- Fixation: Tissue sections are fixed, usually with formalin, to preserve tissue architecture.
- Deparaffinization and Rehydration: Paraffin-embedded sections are deparaffinized in xylene and rehydrated through a graded series of alcohol.
- Staining:
- The sections are immersed in a solution of potassium ferrocyanide mixed with hydrochloric acid. The ferric ions present in the tissue react with ferrocyanide to form the blue pigment.
- The reaction typically takes place at room temperature for about 20 minutes.
- Counterstaining (Optional):
- Sections may be counterstained with nuclear fast red to provide a pink-to-red background, which enhances the contrast of the blue-stained iron deposits.
- Washing and Mounting:
- After staining, the slides are rinsed in distilled water, dehydrated through alcohols, cleared in xylene, and mounted under a coverslip.
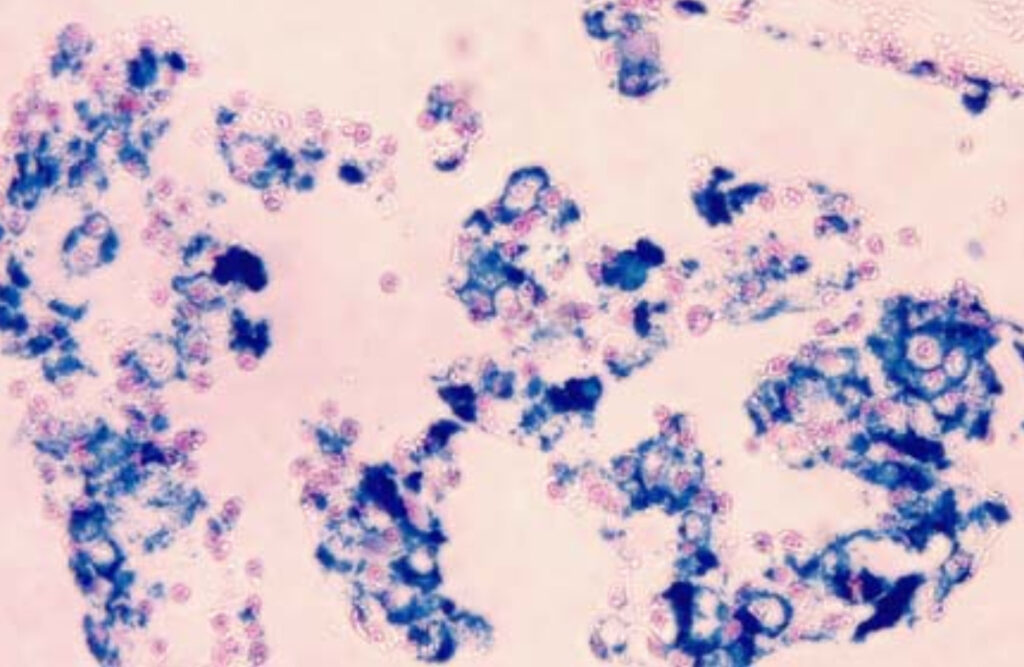
- Blue Areas Indicate the presence of ferric iron, which is stained blue by the reaction between tissue iron and ferrocyanide, marking iron deposits.
- Pink Background: Non-iron-containing components of the tissue are counterstained pink using nuclear fast red, enhancing the visibility of the blue iron deposits.



Leave a Reply