Laboratory Quality Management: An Overview
2.1 Introduction to Quality Management in Laboratories
Quality management systems (QMS) are pivotal to the daily operations of histopathology laboratories and general laboratories, emphasizing the critical nature of structured and robust quality protocols. Laboratories seek accreditation based on their ability to maintain comprehensive quality systems that require full management support and engagement. This support is crucial, as without it, a QMS cannot thrive and becomes a focal point during inspections. All laboratory personnel must be well-versed in QMS processes and prepared through abundant resources that discuss quality management in depth. This chapter introduces the essential elements of quality management and its impact on laboratory operations.
Key Elements of Quality Management
Effective quality management in laboratories encompasses several vital components:
- Governance and Leadership: Strong governance and proactive leadership are essential for fostering an environment where quality management systems can succeed.
- Risk and Opportunity Management: Identifying and managing potential risks and opportunities is critical for maintaining the integrity of laboratory operations.
- Quality System Infrastructure: Establishing and maintaining a robust quality system is fundamental to ensuring reliable and accurate results.
- Human Resources Management: Effective human resources management is crucial for ensuring that all staff are competent, well-trained, and adequately supported.
Quality management is not just a management segment but is integral to the interconnected management systems within an institution. For example, financial systems rely on the high-integrity data produced by quality management systems, enhancing overall institutional operations.
The Role of Biopsies in Histopathology
Biopsies are crucial for supporting or refuting clinical diagnoses through detailed examination and data verification. This process is vital for guiding clinical decisions regarding treatment and providing insights into disease prognosis. The reasons for taking resection specimens include:
- Confirming a diagnosis.
- Ensuring adequate resection margins.
- Determining the extent of tissue involvement.
- Providing predictions on potential outcomes and prognosis.
2.2 Quality Management System (QMS) in Laboratories
A Quality Management System in a laboratory setting is designed to meet predefined quality goals through a structured framework that promotes continuous improvement. The QMS encompasses all aspects of laboratory operations, including interactions with patients, staff management, and facility upkeep. Accurate, reliable, and timely laboratory results are essential for effective healthcare.
Benefits of Implementing a QMS
Implementing a QMS offers numerous advantages:
- Reliability and Consistency: Ensures consistent delivery of accurate laboratory tests.
- Reduction of Errors: Minimizes costly and potentially harmful mistakes.
- Increased Efficiency: Enhances resource utilization and reduces turnaround times.
- Improved Patient Satisfaction: Enhances the overall patient experience and care.
- Technological Advancement: Incorporates new technologies and skills for better healthcare outcomes.
- Enhanced Reputation: Boosts the institution’s image and prepares it for future accreditation assessments.
Establishing a QMS
Critical steps in establishing an effective QMS include:
- Management Responsibility: Quality management must be a top priority for leadership, ensuring resource allocation and support.
- Focus on Patient Care: The system should enhance the delivery of high-quality lab results.
- Objectivity and Integrity: Maintaining impartiality in lab decisions, free from conflicts of interest.
- Clear Documentation: Developing thorough documentation practices for consistency and accuracy.
- Defined Roles and Responsibilities: Clarifying job roles within the laboratory to ensure accountability.
- Staff Training: Providing ongoing education and training to keep staff competent and knowledgeable.
- Effective Communication: Establishing robust communication channels to ensure efficient information flow.
The consequences of incorrect laboratory results can be severe, leading to inappropriate treatments, delayed diagnoses, and unnecessary additional testing, highlighting the critical nature of a well-implemented QMS in laboratory settings.
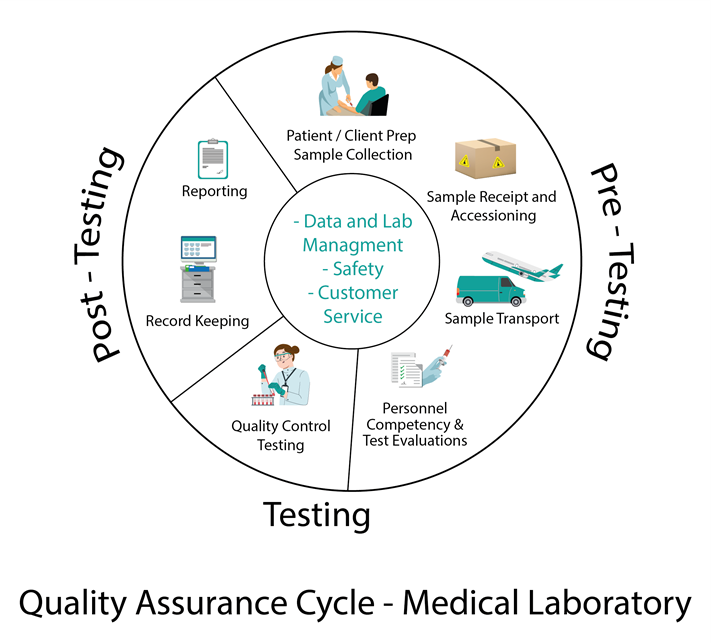
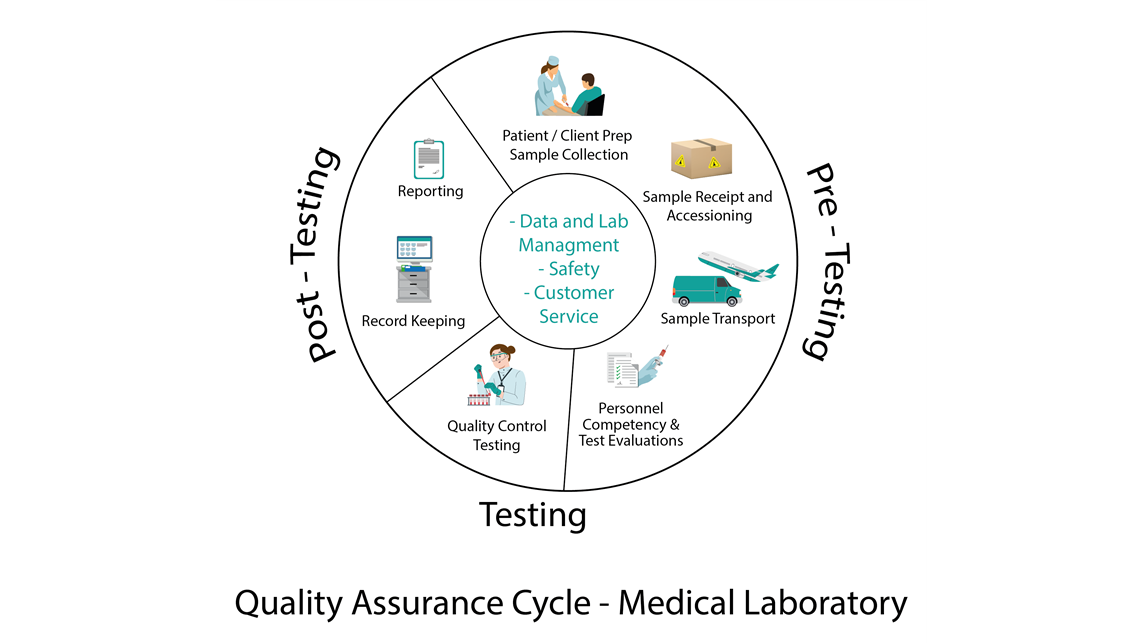
Typical Phases of the Quality Assurance Cycle
1. Plan: Identify the objectives and processes necessary to deliver results based on the specified requirements and the organization’s policies.
2. Do: Implement the processes developed in the planning stage.
3. Check: Monitor and measure processes against the quality policies, objectives, and requirements and report the results.
4. Act: Take actions to improve process performance continually.
This cycle often called the PDCA (Plan-Do-Check-Act) cycle, helps organizations ensure that their processes remain efficient and effective.


Leave a Reply